* Follow the story of virus Rigvir created by Aina Muceniece and become a witness to how Rigvir changes oncology paradigm and helps people.

Prof. Aina Muceniece (1924 – 2010)
Author of ECHO-7 virus discovery
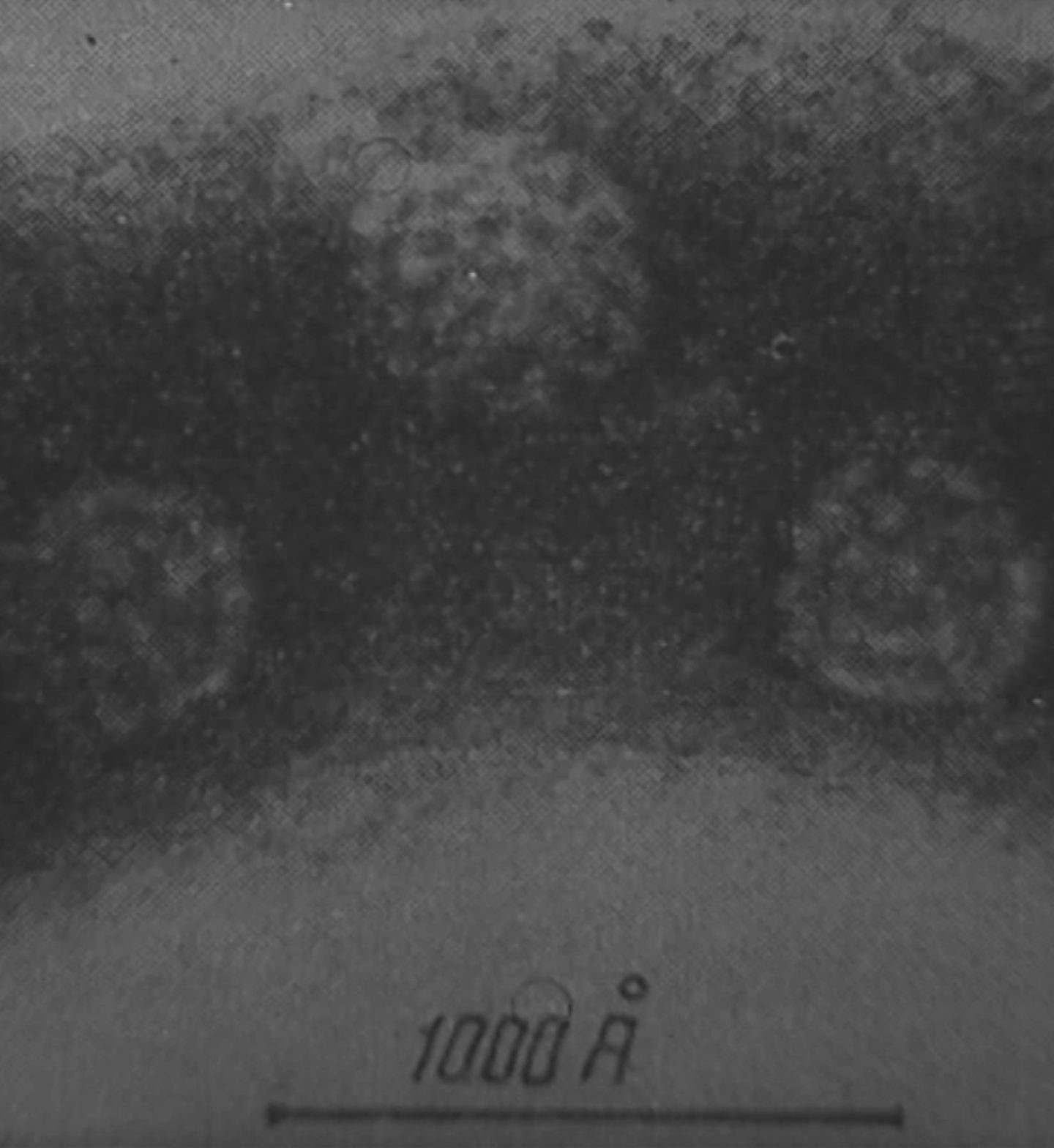
Scientists in the Institute of Microbiology and Virology of the Latvian Academy of Sciences discover that viruses recovered from the intestines of healthy children are capable of destroying tumors.
The Cancer Virotherapy Laboratory is established at the Institute to study this virus phenomenon further under the leadership of Aina Muceniece.

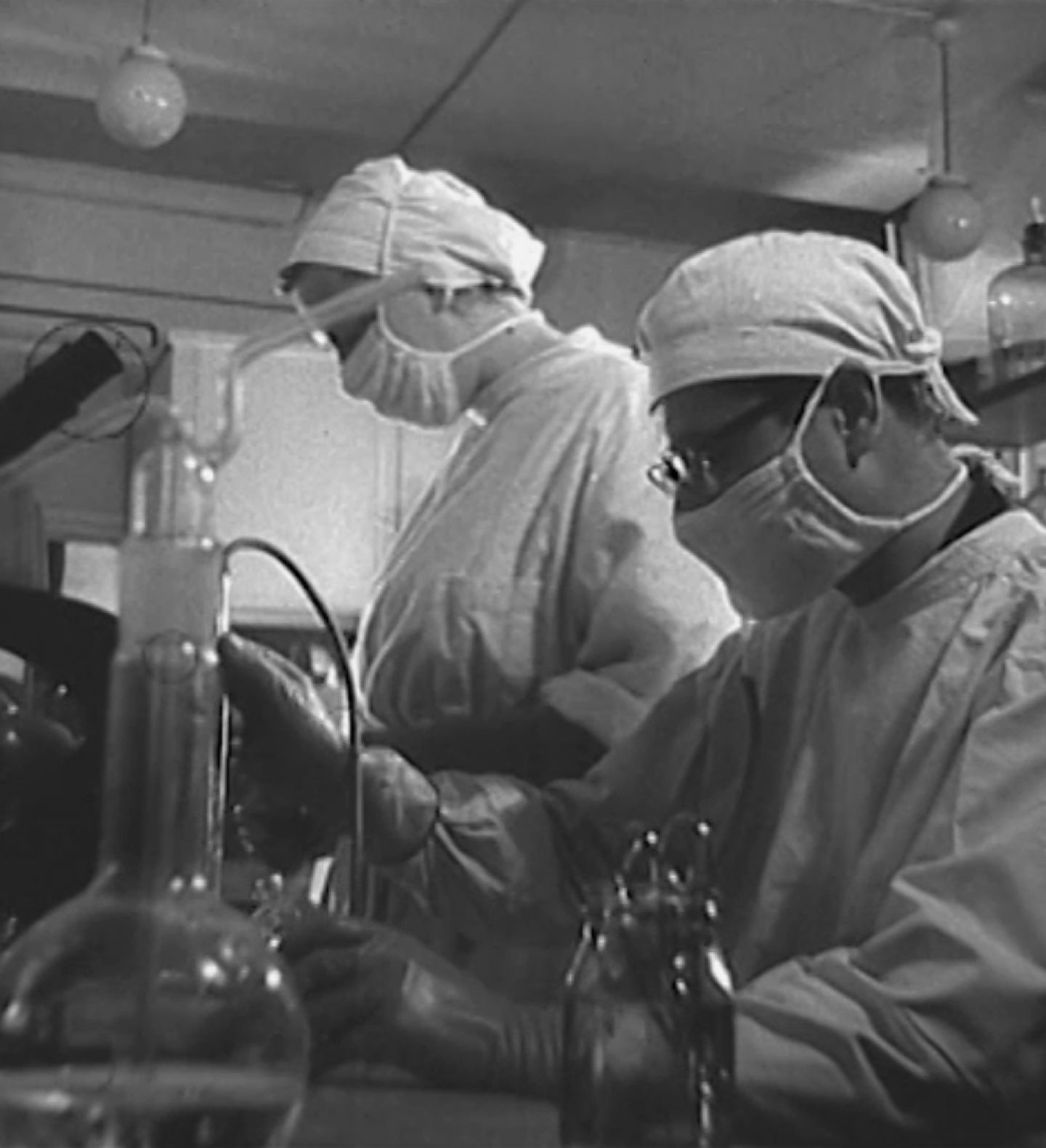
The Ministry of Health Protection (the competent regulatory authority) approves the start of RIGVIR® clinical trials in the 2nd Department of the Oncology Dispensary and the 18th Chemotherapy Department of the Pauls Stradiņš Clinical Hospital in Riga.
Extended RIGVIR® clinical trials start in three Riga hospitals: Latvian Oncology Center (the former Oncology Dispensary), the P. Stradiņš Clinical Hospital, Riga Stradiņš University Institute of Dentistry (at that time AML), and the Saratov Oncology Hospital (Russia). At that time the studies were, however, neither randomised nor double-blind. Over 700 patients are enrolled in these clinical trials until 1991.
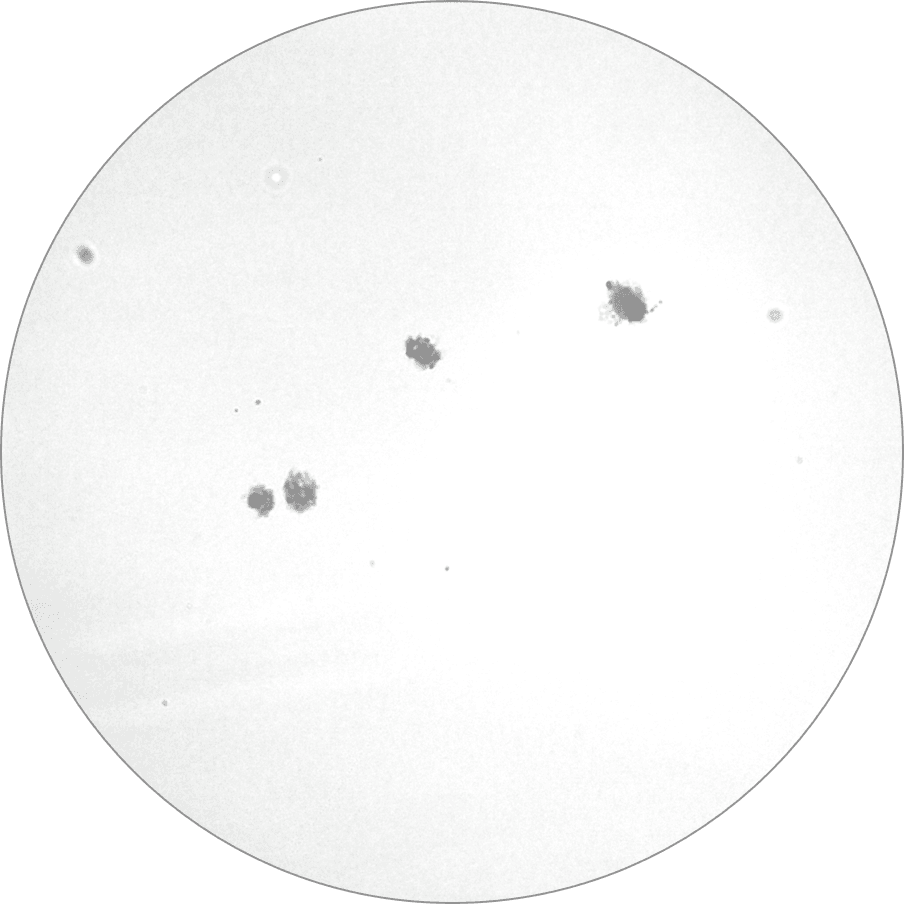

Part of the multicenter and multinational Rigvir clinical trials also start at the All-Union Cancer Research Center in Moscow.
With the destabilization and collapse of Soviet Union the registration and research process of ECHO-7 virus and oncolytic virotherapy is interrupted and halted.

RIGVIR® is registered by the State Agency of Medicines of the Republic of Latvia, thus Rigvir becomes the first approved oncolytic virotherapy medication.
International Virotherapy Center is established in Riga, Latvia. Its mission is to provide virotherapy information for every cancer patient who wants it.

Oncolytic virotherapy with RIGVIR® is included in the list of reimbursable state-funded medical products in Latvia for skin melanoma patients.
Oncolytic virotherapy with RIGVIR® is included in the clinical guidelines of the National Health Service of Latvia entitled “Diagnostics, treatment and dynamic observation of skin cancer and melanoma”.

The first cancer virotherapy excellence center, the Global Virotherapy Cancer Clinic, is opened in Jūrmala, Latvia.
The RIGVIR® marketing authorization holder receives a grant from “Horizon 2020” to support activities for registration in the European Union.


RIGVIR® receives special recommendations for medication registration in EU from European Medicines agency (EMA).
RIGVIR® manufacturer receives intellectual property award “WIPO Enterprise Trophy”.


RIGVIR® manufacturer receives Seal of Excellence award given by European Commission.
Rigvir Group becomes a biotech company.


Rigvir Group assigns the Global Virotherapy Cancer Clinic to a new partner.
Rigvir Group has sold International Virotherapy Center.


Rigvir Group is looking for partners globally
To
finance the commercialization process of a next generation solution for unmet medical needs in
oncology based on the existing product.
At the end of 2019, Rigvir holding successfully attracted investments of EUR 500,000. Investments are attracted from an investor syndicate, including Pharma VC, a company managed by well-known and respectable venture capital investors in Northern Europe. The investment will be used in accordance with the Rigvir science program to strengthen the product's scientific capacity.


Rigvir Holding concludes a deal with Chinese biotech company Sinorda Biomedicine on development and commercialisation of ECHO-7 oncolytic virotherapy cancer medicine in Chinese market and agrees on sales of minority shares in the manufacturing company.
Rigvir's new API (Active pharmaceutical ingredient) center of manufacturing has received GMP (Good Manufacturing Practice) compliance certification for the production of Rigvir virus ECHO-7.


Rigvir Group has signed a license agreement on the manufacturing, registration and sales of Rigvir medicine in the CIS (Commonwealth of Independent States) region countries.
Natural Intelligence Cancer Prevention Project
Rigvir Group has signed a licence agreement with a UK-based company Smart Nanovirus Limited on a fundamentally new approach to cancer prevention, that would allow to identify high cancer risks timely and address them effectively, using the oncolytic virus Rigvir.


Food supplement Rigvir SE is launched on the market
Company Smart Nanovirus Limited that has received a licence to develop the Rigvir virus in the non-medicine sector has successfully launched Rigvir SE on the market on 1st of July, 2023.
More information on www.smartnanovirus.com